Abstract
Background
Acute promyelocytic leukemia (APL) is a unique subtype of acute myeloid leukemia (AML) frequently presenting with a life-threatening coagulopathy. Fatal bleedings such as intracranial haemorrhage are common initial disease manifestations and contribute to a substantial risk of early death in APL. Patients surviving the critical initial disease phase, however, have excellent outcomes with currently used combinations of all-trans retinoic acid (ATRA) in combination with chemotherapy or arsenic trioxide (ATO). Time-points from which overall survival of cancer patients equalize to that of an age-, sex-, and calendar year-matched general population has become an area of increasing interest in clinical trials as well as for direct patient counselling.
Aim
I) To report crude relative survival for de novo APL patients and for the subset of patients surviving the critical initial disease phase and II) to explore associations between well-established clinical risk factors and normalization of survival.
Methods
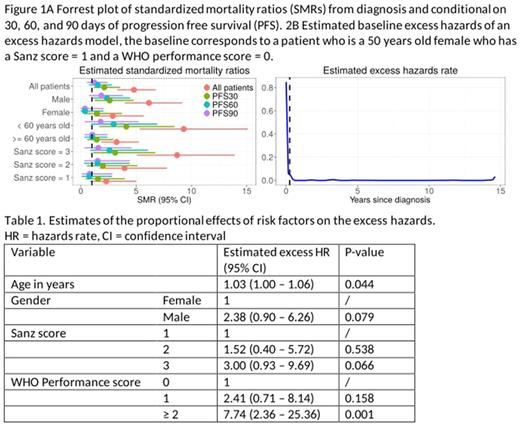
107 de novo APL patients diagnosed between January 2000 and December 2014 (15 years) were identified in the Danish National Acute Leukemia Registry (DNLR), which has coverage of more than 99%. The presence of the PML-RARA fusion gene (t(15;17)(q22;q12)) was documented in all patients. Clinical information was retrieved from DNLR and/or from local medical chart reviews. The survival of an age- sex-, and calendar year-matched background population was constructed using data from the Human Mortality Database. Standardized mortality ratios (SMR) in APL were determined at diagnosis and conditional on reaching progression-free (no death or relapse) survival (PFS) 30 (PFS30), 60 (PFS60), and 90 (PFS90) days after diagnosis. The effect of clinical risk factors (gender, Sanz score, WHO performance status, age ≥/<60 years) on the excess mortality were tested in an excess hazards model. This model focusses on estimating the effect of risk factors on the excess hazards, i.e. the additional hazards on top of the usual hazards rate in a matched population.
Results
Median age was 50 years and male:female ratio was 1.38. 28% of the patients had a Sanz risk score of 1 (platelet count >40x109/L and leukocyte count ≤10x109/L), 38% had a Sanz risk score of 2 (platelet count ≤40x109/L and leukocyte count ≤10x109/L), and 34% had a Sanz risk score of 3 (leukocyte count >10x109/L). At diagnosis, the standardized mortality ratio for APL patients was 4.76 (95% CI 3.25-6.72), but the SMR rapidly decreased to 2.10 (1.15-3.53) and 1.52 (0.73-2.79) after PFS30 and PFS60, respectively. For the APL patients reaching PFS90, the SMR was 1.22 (0.53-2.41). Subgrouping according to baseline risk factors revealed important differences in SMR at baseline but their prognostic relevance diminished after reaching PFS90 (Figure 1A). The small values of the base-line excess hazards from 90 days onwards (Figure 1B) partially confirm the conclusion from the SMR study. Furthermore, the effect of the risk factors on the excess hazards shows an increased risk for older patients, patients with a Sanz score = 3, and patients with a WHO performance score >= 2 (Table 1).
Conclusions
APL patients with de novo disease surviving the critical initial phase have excellent outcomes and survival equivalent to a matched background population already at PFS90.
Severinsen: ARIAD: Research Funding; Celgene: Research Funding; Novartis: Research Funding. Oestgaard: Pfizer: Other: Travel expences; The Danish Cancer Society: Research Funding. Schöllkopf: Karyopharm Therapeutics: Research Funding; Pfizer: Other: Travel expences; Teva: Other: Travel expences. El-Galaly: Roche: Other: Travel funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal